Another SARS-CoV-2 variation that was first identified by researchers in South Africa on November 24 has been named a “variation of concern” (VOC) by the World Health Organization (WHO). New Cases have been distinguished in a developing rundown of nations, including Hong Kong, Israel, Belgium, the United Kingdom, Australia, and Germany.
Proof proposes that the new Omicron (or B.1.1.529) variation might be more contagious than the all-around profoundly contagious Delta variation, with the European Center for Disease Prevention and Control referring to the variation’s “invulnerable getaway potential and possibly expanded contagiousness advantage contrasted with Delta variant.”
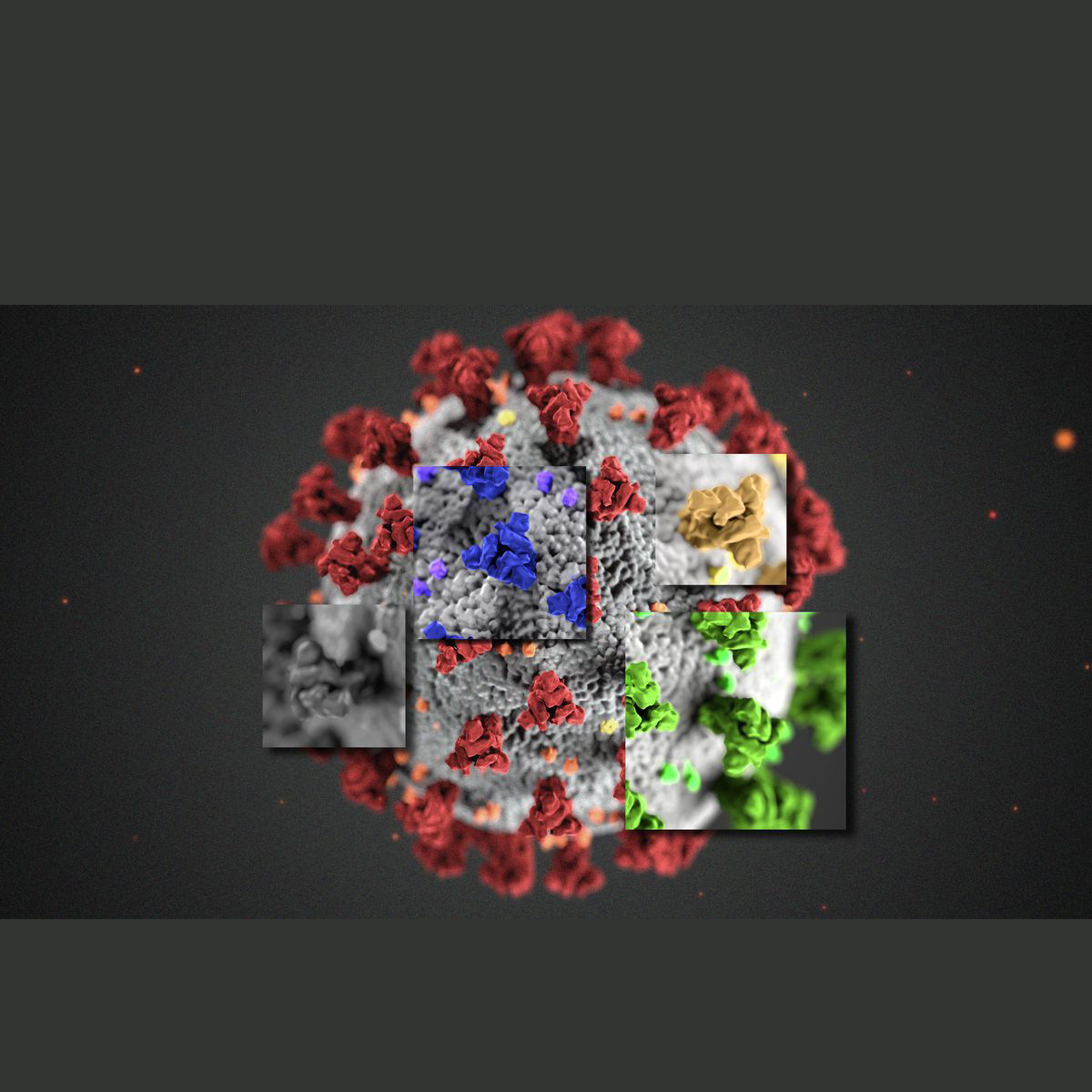
The Omicron variation is described by 30 transformations, three little deletions, and one insertion in the spike protein – the spikes which are outward of the infection and assist it with entering the cell of the host;15 are present in the receptor-binding domain – the piece of the spikes which permit them to tie to a host receptor.
On SARS-CoV-2 Virus Evolution (TAG-VE), The Technical Advisory Group is a group of specialists that intermittently assesses and screens the origination of SARS-CoV-2 and evaluates assuming explicit transformations and mixes of changes adjust the conduct of the infection. The TAG-VE was met on 26 November 2021 to survey the SARS-CoV-2 variation: B.1.1.529.
On 24 November 2021, the B.1.1.529 variation was reported to WHO first time from South Africa. In South Africa, the epidemiological circumstance has been portrayed by three sharp peaks in new cases; the most recent was the Delta variation. Lately, diseases have expanded steeply, harmonizing with the discovery of B.1.1.529 variation. The known initially affirmed B.1.1.529 disease was from a case collected on 9 November 2021.
The most generally utilized antibody vaccines are Moderna, Oxford AstraZeneca, and Pfizer – depend just on getting our immune system to perceive the spike protein part of the SARS-CoV-2 infection as unfamiliar and developing a multitude of resistant cells such as immune cells coordinated at this part of the infection. However, assuming the transformations in Omicron change the spike protein enough that the immune system can presently don’t completely remember it, then, at that point, it might mean there is a level of invulnerable departure for this variation.
It might likewise imply that those depending on regular resistance, invulnerability from a past COVID-19 infection, not something prescribed, may have cause to stress. There is some worry that the changes that Omicron harbors might leave individuals who have been recently infected open to reinfection. As per the WHO, preliminary proof proposed danger of reinfection with this variation, as contrasted and other VOCs.
The U.S. organization of Food and Drug Administration (FDA) has conceded an Emergency Use Authorization (EUA) for REGEN-COV™ (imdevimab and casirivimab) for the treatment of mild to modern COVID-19 infection in pediatric patients (12 years old) and adults with positive consequences of direct SARS-CoV-2 viral testing, and who are at serious danger for movement to extreme COVID-19, including hospitalization or sometimes death.
The U.S. government has made our investigational neutralizer treatment, REGEN-COV, for COVID-19 free to patients who qualify under the Emergency Use Authorization guidelines given by the FDA. An individual with commercial insurance might be dependent upon a co-pay or co-insurance cost for the medication’s organization. Right now, there is no expense for the medication or its organization for patients with Medicare or Medicaid protection.
The Regeneron’s programs for the infectious disease have prompted an authorized medication for the Ebola virus, a crisis-involved medication for COVID-19, and an investigational medication for Middle East Respiratory Syndrome (MERS). For each situation, we have taken a key multi antibody immune response ‘cocktail’ approach.
Coronavirus-related disclosure endeavors began in mid-2020 when we used our VelociSuite® advances to deliver and assess many viruses neutralizing antibodies in our hereditarily designed mice. Knowing from the start that we would adopt a combination strategy, we likewise distinguished comparatively performing antibodies from human COVID-19 survivors to augment the pool of expected candidates. By June, we had chosen and advanced the two potent, complementary, and non-complementary antibodies, imdevimab and casirivimab, into enormous scope producing and clinical trials.
According to the nature of viruses, they mutate over time producing new variant forms. With (at least two) complementary antibodies in a single therapeutic, regardless of whether one antibody has decreased potency because of a specific strain, the danger of the combination losing efficacy is lessened since the infection would have to change in numerous particular areas to avoid the two antibodies. On account of REGEN-COV for COVID-19, casirivimab and imdevimab tie firmly and non-competitively to various, non-overlap portions of the protein’s spike of the SARS-CoV-2 infection, in this way impeding the infection’s capacity to infect normal cells.
On Tuesday, it was stated that Regeneron Pharmaceuticals Inc’s COVID-19 immunizer medication could be less successful against Omicron, adding to alarm about the efficacy of available treatments after Moderna’s top manager raised comparable worries about the organization’s immunization.
Worldwide business sectors tumbled after remarks from Moderna’s CEO revived concerns that the variation might burden a beginning worldwide financial recuperation.
Given its investigation of Omicron’s mutation, “there might be diminished balance action of both vaccine-induced and monoclonal antibody conveyed immunity,” further Regeneron said, adding that the examination incorporated its COVID-19 antibody cocktail that is REGEN-COV.
The organization said it was doing an additional study to measure the potential effect utilizing the variation’s genetic sequence.
One of the antibodies utilized in the treatment will probably endure a shot, the other less along these lines, CEO Len Schleifer said in a CNBC meet.
“Just like vaccines will have to adapt, we’re probably going to have to adapt our monoclonals constantly.”
Eli Lilly and Co, which makes an equal monoclonal antibody treatment, is additionally attempting to comprehend neutralization activities of its treatments on Omicron, the organization told Reuters in an online statement via email.
Regeneron shares decreased around 3% in daytime exchanging, while those of Lilly fell 2.5%.
Rival Vir Biotechnology Inc said that depending on the Omicron sequence, its immune response treatment (antibody), sotrovimab, will probably keep up with potency against the variant gene.
The organization is “attempting to affirm this in the lab as an issue of urgency,” it added.
Gilead Inc said it trusted its intravenous treatment, Remdesivir, at present the main antiviral supported for treating COVID-19, will keep on being effective against, as of now, recognized variations, including Omicron.
On Tuesday, The U.S. FDA said it assessed the effectiveness of the approved COVID-19 immunizations vaccine against the Omicron variant and was hoping to have more data in the following many weeks.
Also Read: Moderna Says It Might Release A Modified COVID-19 Vaccine For Omicron Variant By 2022