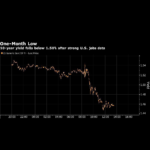
Payrolls Are Late For Shows Even When There Is Bad News, The Stock Market Performs Well
Shareholders traded on macroeconomic factors rather than the Governmental Treasury’s preconceptions for stimulus spending after poor November payrolls data was released, reversing a months-long trend in which investors sold on economic fundamentals rather than the Federal Reserve’s expectations for economic stimulus. The 60-day correlation between S&P 500 e-mini futures and the Citi Economic Shock Index, … Read More